University of California, San Diego
Mechanical and Aerospace Engineering Department
del Álamo’s Research Group

Intraventricular Flow

Turbulent Wall Flows
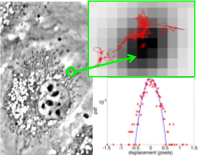
The intracellular domain consists of a semi-dilute filamentous network embedded in a fluid phase. The microrheological properties of this coupled multiphase system play a determinant role in many cellular functions, ranging from cell migration (involved in cancer spreading, immune response, etc.) to the ability of the cell to convert mechanical stimuli into chemical activity (involved in stem cell differentiation, epithelial and endothelial response to flow, etc.).
Current experimental methods estimate these properties by measuring the resistance that intracellular probing particles with diameters ∼ 0.1 − 1μm experience as they move through the cell. A crucial step in these methods is to connect the measured resistance to the underlying cellular properties, which is usually accomplished by assuming that the particles experience Stokes drag. The assumption of Stokes flow is pivotal in microrheology but it breaks in live cells due to the structural complexity of the intracellular domain. Tissue and cultured cells have marked anisotropic structural characteristics: all cells in the same tissue/culture are elongated along a common direction and their intracellular filaments form bundles aligned in the same direction.
Our research aims to address these limitations by 1) providing the missing basic knowledge about the hydrodynamics of microrheological probes in anisotropic semi-dilute networks, 2) employ this new knowledge to develop novel directional microrheology methods that can measure the viscoelastic properties of these networks, and 3) apply these new methods to quantify the anisotropic microrheological properties of live cells, in collaboration with Prof. Chien’s Vascular Bioengineering Lab at UCSD.
Cytoplasm Microrheology




Cellular Locomotion
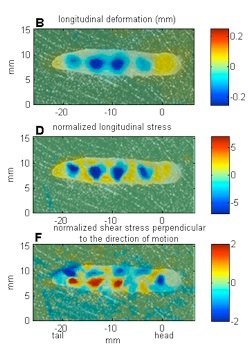
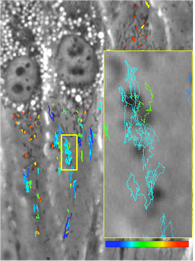
Research on the adhesive locomotion of terrestrial gastropods provides a source of guidance for the design of soft biomimetic robots that can perform functions currently not achievable by conventional rigid vehicles. The locomotion of terrestrial gastropods is driven by a train of periodic muscle contractions (pedal waves) and relaxations (interwaves) that propagate from their tails to their heads. These ventral waves interact with a thin layer of mucus secreted by the animal that transmits propulsive forces to the ground. The exact mechanism by which these propulsive forces are generated is still a matter of controversy. Specifically, the exact role played by the complex rheological and adhesive properties of the mucus is not clear. To provide quantitative data that could shed light on this question, we measure, with high temporal and spatial resolution, the propulsive forces that terrestrial gastropods generate while crawling on smooth flat surfaces, in collaboration with Profs. Rodriguez-Rodriguez at Universidad Carlos III Madrid and Lasheras at MAE. Analysis of the traction forces reveals that the kinematics of the pedal waves is far more complex than previously thought, showing significant spatial variation (acceleration/deceleration) as the waves move from the tail to the head of the animal.
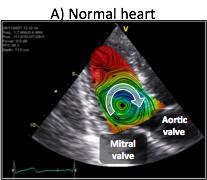
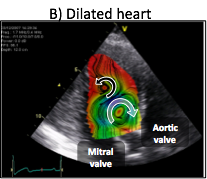
Blood flow generation by the heart is the result of synchronized electromechanical myocardial events and fluid dynamics processes. Interactions between blood flow and the myocardium elicit the continuous remodeling of the heart, leading to flow patterns that minimize energy losses. The flow pattern in the normal left ventricle consists of a large diastolic vortex that channels the transit of blood towards the outflow tract. In a failing left ventricle, progressive adverse remodeling leads to abnormal flow patterns that are less efficient in channeling blood transit, and which may contribute further to the progression of heart failure. Thus, a deeper understanding of blood flow dynamics in normal and diseased left ventricles may provide insight on the pathophysiology of heart failure, leading to earlier diagnosis and improved treatment strategies of this syndrome.
Recent years have witnessed a surge of interest in studying the role of flow patterns in blood transport inside the left ventricle. Despite significant advances, we still understand poorly how the fluid dynamical processes are synchronized with the electromechanical events to generate blood flow. The goal of our research is to understand the dependence of the time evolution of the diastolic vortices on the duration of the left-ventricular filling phases, and to determine how this dependence affects blood flow transport and global ventricular function.
In collaboration with Dr. Javier Bermejo at Hospital Gregorio Marañón in Madrid, we have developed novel echocardiographic modality to measure two-dimensional bi-directional time-resolved flow maps in the left ventricle. 2D+t color-Doppler flow mapping is fast, clinically-compliant and does not require complex training. The ultrasound acquisitions required to obtain a time-resolved flow map in a patient can be easily completed in less than 5 minutes, and the ensuing offline processing is performed in approximately 2-3 minutes using parallelized in-house routines running on multi-core, off-the- shelf desktop computers.
We are now collaborating with Prof. Shadden at IIT and a team of cardiologists at the UCSD Medical Center, led by Dr. DeMaria and Dr. Kahn, to determine the time evolution of diastolic vortices and characterize how the synchrony between these vortices and ventricular wall motion affects the transit of blood inside the left ventricle. Better knowledge of this dependence will increase our understanding of the benefits of cardiac resynchronization therapies by improving patient selection and enabling LV contraction to take place under the most favorable hemodynamic conditions.
Gastropod Locomotion
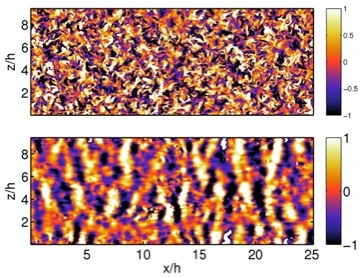
Turbulent wall flows are a challenging research subject with applications ranging from drag reduction of vehicles to mixing and dispersion in the atmosphere and the oceans. Understanding the interactions between the outer, inertia-dominated layer and the near-wall, viscosity-dominated layer of these flows are particularly interesting. Inner -> outer interactions are altered by the hydraulically-rough surfaces often found in engineering and geophysical flows, and can be exploited to design flow control strategies through actuators located at the wall. Outer -> inner interactions are largely modified under stable stratification (e.g. in the nocturnal atmospheric boundary layer and the ocean).
In collaboration with Prof. Manuel Garcia-Villalba at Universidad Carlos III Madrid, we are currently investigating how stable stratification modifies turbulence near a wall using large-scale simulations and linear stability theory. From a fundamental point of view, these flows constitute a natural system in which the outer flow region is largely modified while the near-wall region remains mostly unaltered. Thus, they provide a framework to study the interaction between these regions in the neutral case.



Mechanics of Soft-Bodied Organisms