|
Mckittrick group |
Department of Mechanical and Aerospace EngineeringMaterials Science and Engineering Program |
|
ZnO bandgap engineering |
|
Our main motivation for exploring ZnO is its incorporation into short-wavelength optical devices. ZnO has a band gap of 3.37 eV, which corresponds to emission in the UV region. This band gap is very close to that of GaN (3.39eV), and GaN has been the subject of much research over the past years, even being incorporated into the recent Blu-Ray drives. However, ZnO has some significant advantages in its large free exciton binding energy (60 meV compared to 21-25 meV for GaN) that allows for efficient excitonic emission at room temperature1.
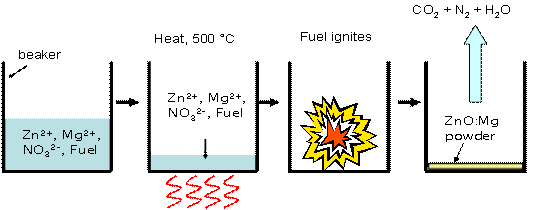
The McKittrick lab has been focused on solution-based methods for synthesis of polycrystalline ZnO alloys, as they are relatively inexpensive and economical. Combustion synthesis is one of these methods, which utilizes a water-soluble fuel which combusts and forces dopant atoms into the ZnO crystal lattice. Figure 1 shows a typical reaction procedure using Mg as the dopant. The products made through combustion synthesis are nanoparticles which are relatively well dispersed, as can be seen in Figure 2. |
|
Figure 1: Combustion Synthesis Procedure |
|
Maintained by: Ekaterina Evdokimenko Last Updated April 3, 2013 |
|
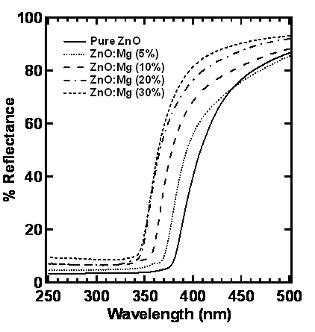
Successful band gap modulation was also obtained in combustion-synthesized products after doping with Mg ions. Figure 3 shows reflectance spectra of ZnO:Mg samples; the location of the drop-off of reflectance is a rough indicator of the band gap.
As ZnO alloys are synthesized, the structural properties are analyzed using x-ray diffraction (XRD) and scanning-electron microscopy and energy-dispersive spectroscopy (SEM-EDS). The optical properties are analyzed through reflectance spectroscopy and photoluminescence spectroscopy.
1Wang, Zhong L. “Zinc Oxide Nanostructures: Growth, Properties and Applications.” J. Phys.: Condens. Matter 16 (2004):R829-R858. |

|
Figure 3: Reflectance Spectra of ZnO:Mg Powders |
|
Figure 2: SEM Micrograph of ZnO Particles |
|
Written by: Steven Shimizu |