|
Mckittrick group |
Department of Mechanical and Aerospace EngineeringMaterials Science and Engineering Program |
|
Maintained by: Ekaterina Evdokimenko Last Updated April 3, 2013 |
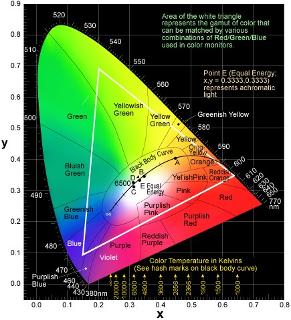
Luminescent materials such as phosphors are materials that emit light (infrared to ultraviolet) under external energy excitation. The incident energy, in the form of high energy electron, photons, or electric field, can then be re-emitted in the form of electromagnetic radiation. For certain phosphors, this radiation fall in the visible spectrum. In order to categorize the radiation in the visible spectrum, the Commission Internationale de I'Eclairage (CIE) standardized the method by which color is quantified by establishing the CIE chromaticity diagram in 1931
CIE Chromaticity Diagram
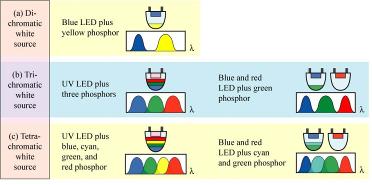
Any three color coordinates on the diagram produces a triangle, in which the colors inside that triangle can be produced by mixing the three colors given by the vertices of the triangle. Pure colors, such as the single wavelength emitted by a laser, are located at the edges of the diagram. Near the center of the diagram is white light that is produced by the sun. The line that starts in the red and curves in through the white section of the CIE diagram represents the black body curve. To achieve a full color display, red, green, and blue emitting phosphors need to be chosen that are nearly saturated to produce a wide range of colors, or color gamut. White LEDs, therefore, have traditionally been fabricated by combining different monochromatic light sources as outlined below.
Traditional oxide phosphors typically consist of an inert host lattice that is doped with activator ions, usually transition (3d) or rare earth (4f) metals. The host lattice is transparent to the incident radiation and the activator is excited to emit photons.
The energy of the activator ion is raised to an excited state by promoting one or more electrons from their ground state to higher energy levels. As the electron returns to the ground state, a photon is radiatively emitted. Nonradiative emissions can occur when the activator emits phonons, which are lattice vibrations that transport energy in the form of heat.
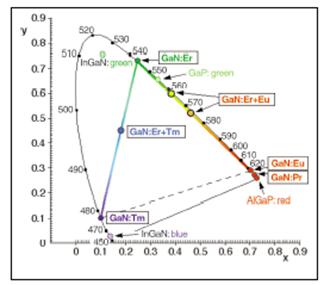
Our research aims to find suitable rare-earth activators and nitride host combinations to produce the desired luminescent material in the red, green or blue-emission range as powders and thin films. Elements such as Eu, Er, Tb and Tm have been shown as red, green and blue emitters in GaN thin films made by MBE2 and AlN powders3 respectively. In the past, combustion synthesis have been used to synthesized rare-earth activated oxide phosphors with great success. A solution-based method is the current method used in the McKittrick lab to synthesized rare-earth activated AlN and GaN powders, where a sequential conversion from water-soluble nitrate precursors to a series of intermediate compounds and nitrides allows for an intimate mixture of rare-earth activator into the nitride host. Following successful conversion, the powders and/or thin films can be characterized by room temperature photoluminescence spectroscopy (PL), or cathodoluminescence spectroscopy (CL) in our lab.
|
Schematic courtesy: E.F. Schubert, Light-Emitting Diodes, Cambridge University Press (www.lightemittingdiodes.org). |
Reference:
1. G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, Berlin (1994).
2. Diagram source: Steckl et al., “Rare-earth doped GaN:Growth, Properties and Fabrication of ELDs,” IEEE Journal of Selected Topics in Quantum Electronics, 8 [4] 749 (2002).
3. B. Han, K.C. Mishra, M. Raukas, K. Klinedinst, J. Tao, and J.B. Talbot, “A Study of Luminescence from Tm3+, Tb3+, and Eu3+ in AlN Powder,” J. Electrochem. Soc., 154 (9), p. J262 (2007).
|
Luminescent materials
|